The chemical properties of purines and pyrimidines, their structure & functions and other interesting facts are presented in the article.
The purines and pyrimidines form an important part of DNA and RNA – which are the blueprints of genomes. Base pairing between nucleotides results into the formation of bonds which play a crucial role in chemical reactions. Here is more on the nucleotides, purines and pyrimidines.
What Are Purines and Pyrimidines?
The purines and pyrimidines are nucleotides which form the building blocks of nucleic acids. Some of the examples of purines are as follows.
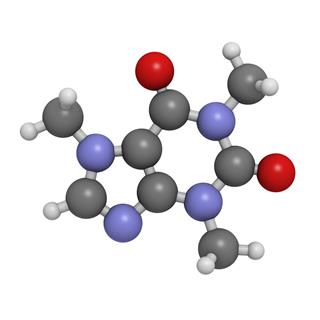
- Adenine: 9H-purin-6-amine (IUPAC Name), 6-aminopurine (Other Name)
- Guanine: 2-amino-1H-purin-6(9H)-one (IUPAC Name), 2-amino-6-hydroxypurine (Other Name)
- Xanthine: 3,7-Dihydropurine-2,6-dione (IUPAC Name), 1H-Purine-2,6-diol (Other Name)
- Hypoxanthine: 1H-purin-6(9H)-one (IUPAC Name), 6-oxypurine (Other Name)
The examples of pyrimidines of common occurrence are listed below.
- Thymine: 5-Methylpyrimidine-2,4(1H,3H)-dione (IUPAC Name), 5-methyluracil (Other Name)
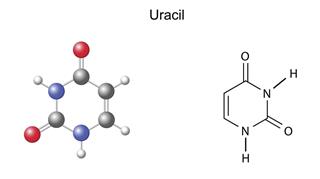
- Uracil: Pyrimidine-2,4(1H,3H)-dione (IUPAC Name), 2,4-dihydroxypyrimidine (Other Name)
- Cytosine: 4-aminopyrimidin-2(1H)-one (IUPAC Name), 4-amino-1H-pyrimidine-2-one (Other Name)
The term, purine was coined by Emil Fischer, a German chemist, in 1884. Purines that are biologically synthesized as nucleosides are produced by means of metabolic pathways of different organisms. Purines are found not just in the molecules of DNA and RNA, but also in ATP, NADH, GTP, cyclic AMP and co-enzyme A; purines are found mostly in meat products. Plant-based foods do not contain large amounts of purines. Sardines, anchovies, sweetbreads, etc. are the rich sources of purines. The high intake of meat, which contains purines, is associated with gout. Therefore, one should consider the option of having a low purine diet.
Chemical properties of pyrimidines are similar to that of pyridines. Pyrimidines are the compounds produced through the process of organic synthesis. One of the methods through which pyrimidines can be synthesized artificially is the Biginelli reaction.
Pyrimidines are aromatic heterocyclic organic compounds that consist of a pyrimidine ring which is fused to a ring of imidazole. Molecules like guanine and adenine are derivatives of a class called purine – which is not a real molecule in itself. In short, these derivatives are manifestation of a ‘virtual’ class called purine. Adenine and guanine are the purines which participate in DNA synthesis through high-energy bonding. Purines and pyrimidines participate in the growth of RNA and DNA through a process called transcription or DNA replication. Short-term energy storage is also one of the functions of these nucleotides.
In the process of nucleotide synthesis, purines and pyrimidines form hydrogen bonds with each other. The structure of nucleotides is such that three hydrogen bonds are formed between guanine and cytosine while adenine and thymine form two hydrogen bonds with each other. Such type of bonding is referred as base pairing.
Difference between Purines and Pyrimidines
The difference between these two nucleotides is that there is just one carbon-ring present in pyrimidines. In case of purines, the carbon-rings are two in number.
- Purines have higher melting and boiling points than pyrimidines. The reason behind this difference in melting and boiling points is that the molecules of purines are complex and heavy. Purines participate in greater number of molecular reactions in comparison to pyrimidines.
- Purines are known to act as precursor molecules in the synthesis of chemical compounds like theophylline, theobromine, caffeine, etc. Pyrimidines are not known to function as precursor molecules.
The above article deals with different aspects of purines and pyrimidines. Hope the details presented above helped you to understand the functions and differences between these two nucleotides.