The electron transport chain is an essential metabolic pathway that produces energy by carrying out a series of redox reactions. This BiologyWise article provides a simple explanation of this pathway.
Did You Know?
One cycle of the electron transport chain yields about 30 molecules of ATP (Adenosine triphosphate) as compared to the 2 molecules produced each via glycolysis and the citric acid cycle.
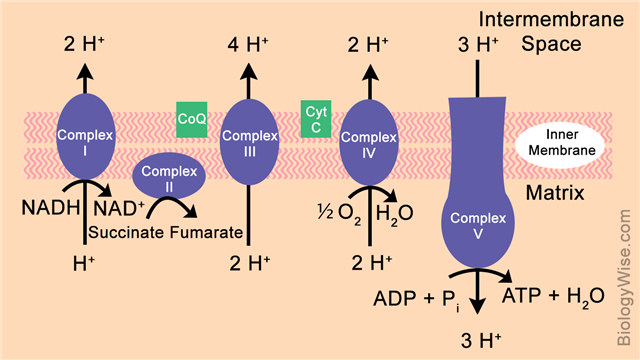
The electron transport chain is made up of a series of spatially separated enzyme complexes that transfer electrons from electron donors to electron receptors via sets of redox reactions. This is also accompanied by a transfer of protons (H+ ions) across the membrane. This leads to the development of an electrochemical proton gradient across the membrane that activates the ATP synthase proton pump, thereby, driving the generation of ATP molecules (energy). The cycle ends by the absorption of electrons by oxygen molecules.
In eukaryotic organisms, the electron transport chain is found embedded in the inner membrane of the mitochondria, in bacteria it is found in the cell membrane, and in case of plant cells, it is present in the thylakoid membrane of the chloroplasts.
In chloroplasts, photons from light are used produce the proton gradient; whereas, in the mitochondria and bacterial cells, the conversions occurring in the enzyme complexes, generate the proton gradient.
Overview of Electron Transport Chain
This pathway is the most efficient method of producing energy. The initial substrates for this cycle are the end products obtained from other pathways. Pyruvate, obtained from glycolysis, is taken up by the mitochondria, where it is oxidized via the Krebs/citric acid cycle. The substrates required for the pathway are NADH (nicotinamide adenine dinucleotide), succinate, and molecular oxygen.
NADH acts as the first electron donor, and gets oxidized to NAD+ by enzyme complex I, accompanied by the release of a proton out of the matrix. The electron is then transported to complex II, which brings about the conversion of succinate to fumarate. Molecular oxygen (O2) acts as an electron acceptor in complex IV, and gets converted to a water molecule (H2O). Each enzyme complex carries out the transport of electrons accompanied by the release of protons in the intermembrane space.
The accumulation of protons outside the membrane gives rise to a proton gradient. This high concentration of protons initiates the process of chemiosmosis, and activates the ATP synthase complex. Chemiosmosis refers to the generation of an electrical as well as a pH potential across a membrane due to large difference in proton concentrations. The activated ATP synthase utilizes this potential, and acts as a proton pump to restore concentration balance. While pumping the proton back into the matrix, it also conducts the phosphorylation of ADP (Adenosine Diphosphate) to yield ATP molecules.
Enzyme Complexes of Electron Transport Chain
Complex I – NADH-coenzyme Q oxidoreductase
The reduced coenzyme NADH binds to this complex, and functions to reduce coenzyme Q10. This reaction donates electrons, which are then transferred through this complex using FMN (Flavin mononucleotide) and a series of Fe-S (Iron-sulpur) clusters. The transport of these electrons brings about the transfer of protons across the membrane into the intermembrane space.
Complex II – Succinate-Q oxidoreductase
This complex acts on the succinate produced by the citric acid cycle, and converts it to fumarate. This reaction is driven by the reduction and oxidation of FAD (Flavin adenine dinucleotide) along with the help of a series of Fe-S clusters. These reactions also drive the redox reactions of quinone. These sets of reactions help in transporting the electrons to the third enzyme complex.
Complex III – Q-cytochrome c oxidoreductase
This complex oxidizes ubiquinol and also reduces two molecules of cytochrome-c. The electron is transported via these reactions onto complex IV accompanied by the release of protons.
Complex IV – ytochrome c oxidase
The received electron is received by a molecular oxygen to yield a water molecule. This conversion occurs in the presence of Copper (Cu) ions, and drives the oxidation of the reduced cytochrome-c. Protons are pumped out during the course of this reaction.
ATP Synthase
The protons produced from the initial oxidation of the NADH molecule, and their presence in the intermembrane space gives rise to a potential gradient. It is utilized by this complex to transport the protons back into the matrix. The transport itself also generates energy that is used to achieve phosphorylation of the ADP molecules to form ATP.
Any anomalies or defects in any of the components that constitute the electron transport chain leads to the development of a vast array of developmental, neurological, and physical disorders.